AMPTRAN Motor Corporation
Advanced Vehicle Testing Lab
|
THE SOLUTION: Over the past 10 years, our team has been working on lithium-air battery, which is estimated could hold 5 to 10 times as much energy as a lithium-ion battery of the same weight and double the amount for the same volume. In theory, the energy density could be comparable to that of gasoline. Most attempts to improve the design of lithium ion batteries have tackled the problem at the macroscopic scale, but work is now focusing on the nanoscale. Nanomaterials were slow to enter the field of energy storage because the effective increase in the electrodes’ surface area raised the risk of secondary reactions involving electrolyte decomposition.
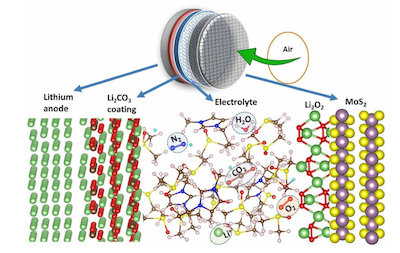
The hunt is under way for nanomaterials that can undergo conversion reactions involved multiple electrons at high potential, for use as cathode materials. In collaboration with our partners, NASA Ames Research Center, Lithium Air Industries brings unique expertise in nanotechnology to solve some fundamental problems in the development of Lithium Air battery technologies. The newly developed Lithium Air Technology will be used to power a typical electric car such as a Chevrolet Bolt to go for 500-600 miles per single charge. Today, the Chevrolet Bolt with lithium ion battery can go only 238 miles per single charge. The Lithium Air Battery technology developed by our team is based on proprietary Nanocomposite Technologies. In practice, our research team has developed an patented Li–air batteries with a specific energy of ~6.35 MJ/kg at the cell level. This is about 5 times greater than that of a commercial lithium-ion battery, and is sufficient to run a 3,600 lb electric car for ~500 miles on one charge using only 200 lbs of Lithium air batteries. This is compared with the 4,500 lb Tesla goes only 300 miles with 1500 lb lithium ion battery. The lithium–air battery (Li–air) is a metal–air electrochemical cell or battery chemistry that uses oxidation of lithium at the anode and reduction of oxygen at the cathode to induce a current flow.
Pairing lithium and ambient oxygen can theoretically lead to electrochemical cells with the highest possible specific energy. Indeed, the theoretical specific energy of a non-aqueous Li–air battery, in the charged state with Li2O2 product and excluding the oxygen mass, is ~40.1 MJ/kg. This is comparable to the theoretical specific energy of gasoline, ~46.8 MJ/kg. 
|
|---|
Copyright © 2011-2021 Lithium Air Industries-. All rights reserved. |